Medical Device ERP: How YoctoERP Streamlines Compliance and Operations
Medical device companies operate under one of the strictest regulatory environments in the world. Every pulse oximeter, stethoscope, or diagnostic device requires accurate inventory tracking, documented quality inspections, and full regulatory compliance. A single mistake—missed inspection, mismanaged stock, or incomplete documentation—can trigger costly audits, recalls, or even product bans. That’s why a dedicated medical device ERP is not just useful—it’s essential.
YoctoERP is designed specifically for medical device companies, combining procurement, inventory, quality control, and compliance into one seamless system. Here’s why it stands out.
Why a Medical Device ERP is Critical
Unlike generic ERP systems, a medical device ERP must meet industry-specific demands:
- Traceable Inventory: Every batch of devices is tracked from supplier to warehouse to customer. This ensures rapid response during recalls or regulatory audits.
- Mandatory Quality Checks: CE marking verification, calibration certificates, and functional tests are documented in the system. This avoids human error and ensures regulatory compliance.
- Audit-Ready Records: All procurement, inspection, and stock movement activities are logged automatically. You can produce reports in seconds instead of weeks.
YoctoERP addresses these challenges with workflows tailored for real-world operations, not just theory.
Streamlined Procurement for Medical Devices
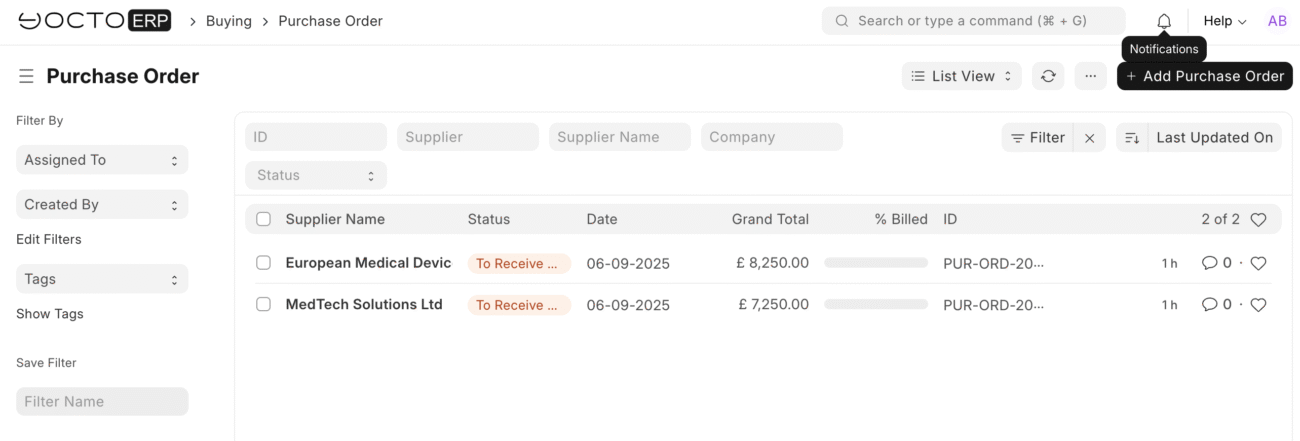
Procurement is the first step in the supply chain, and mistakes here ripple through your operations. YoctoERP handles it efficiently:
- Request for Quotation (RFQ): Create and send RFQs to multiple suppliers. Include device-specific details like model, batch size, and delivery timelines.
- Supplier Quotation Management: Receive supplier quotes and compare prices, lead times, and terms within YoctoERP.
- Purchase Orders: Convert approved quotations into POs with one click, linking each order to specific suppliers, items, and batches.
This ensures that your procurement is organized, traceable, and ready for regulatory audits.
Accurate Goods Receipt
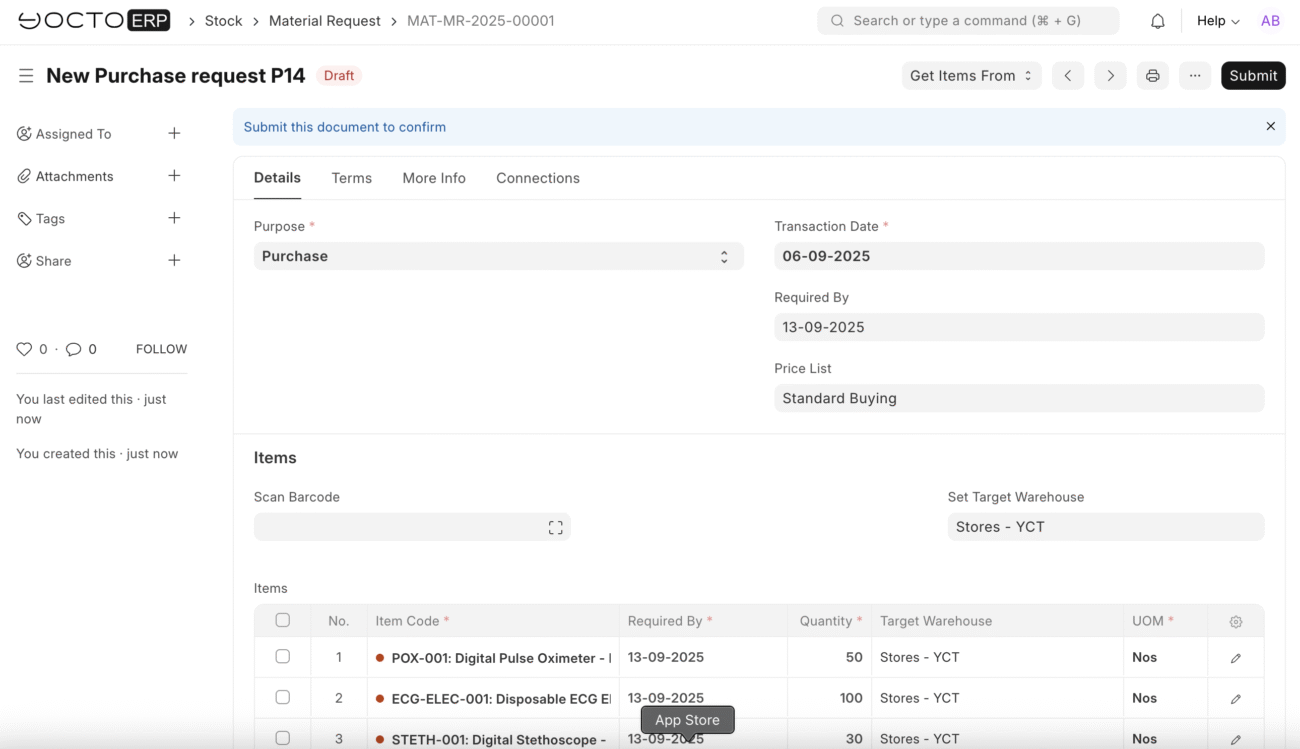
When shipments arrive, YoctoERP makes receiving goods simple and error-proof:
- Confirm quantities against purchase orders.
- Record partial deliveries or discrepancies instantly.
- Automatically update stock levels in real time.
For example, if you order 50 pulse oximeters from MedTech Solutions and only 45 arrive, YoctoERP flags the shortage and links it directly to the original purchase order. No lost paperwork, no guesswork.
Quality Inspections Built for Compliance
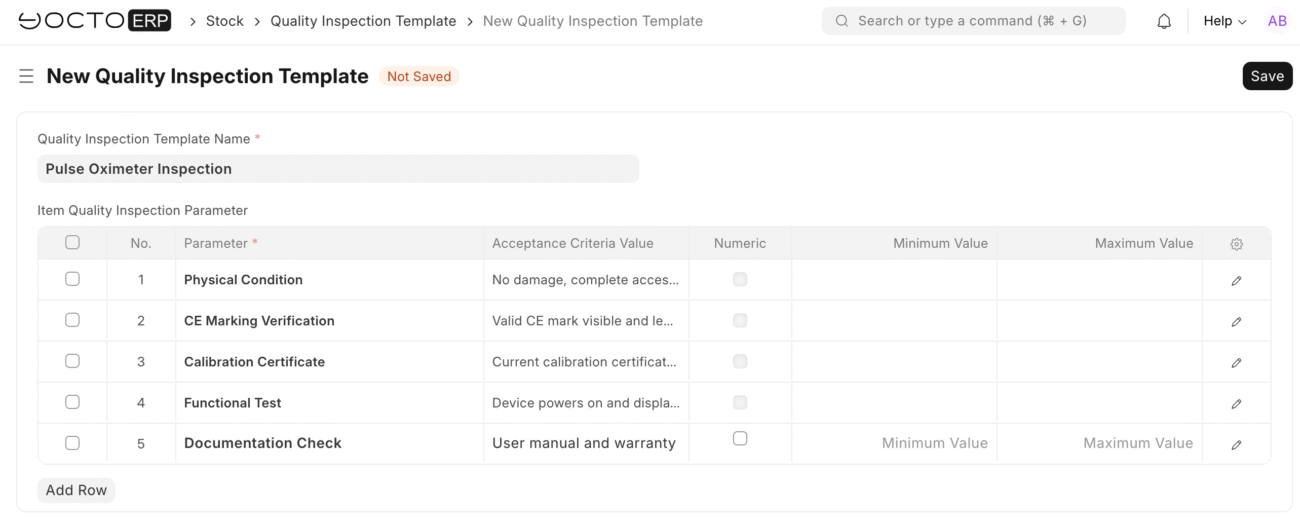
Medical device regulations require that every item undergoes documented quality inspection before entering inventory. YoctoERP streamlines this process:
- Inspection Templates: Use pre-configured templates for each device type. Templates include CE marking, calibration certificates, and functional testing steps.
- Incoming Inspections: Link inspections directly to received purchase receipts. Record sample sizes, test results, and notes.
- Approval or Quarantine: Items that pass move automatically into main inventory; failing items remain in quarantine until resolved.
This workflow reduces human error, ensures consistency, and guarantees compliance with industry standards.
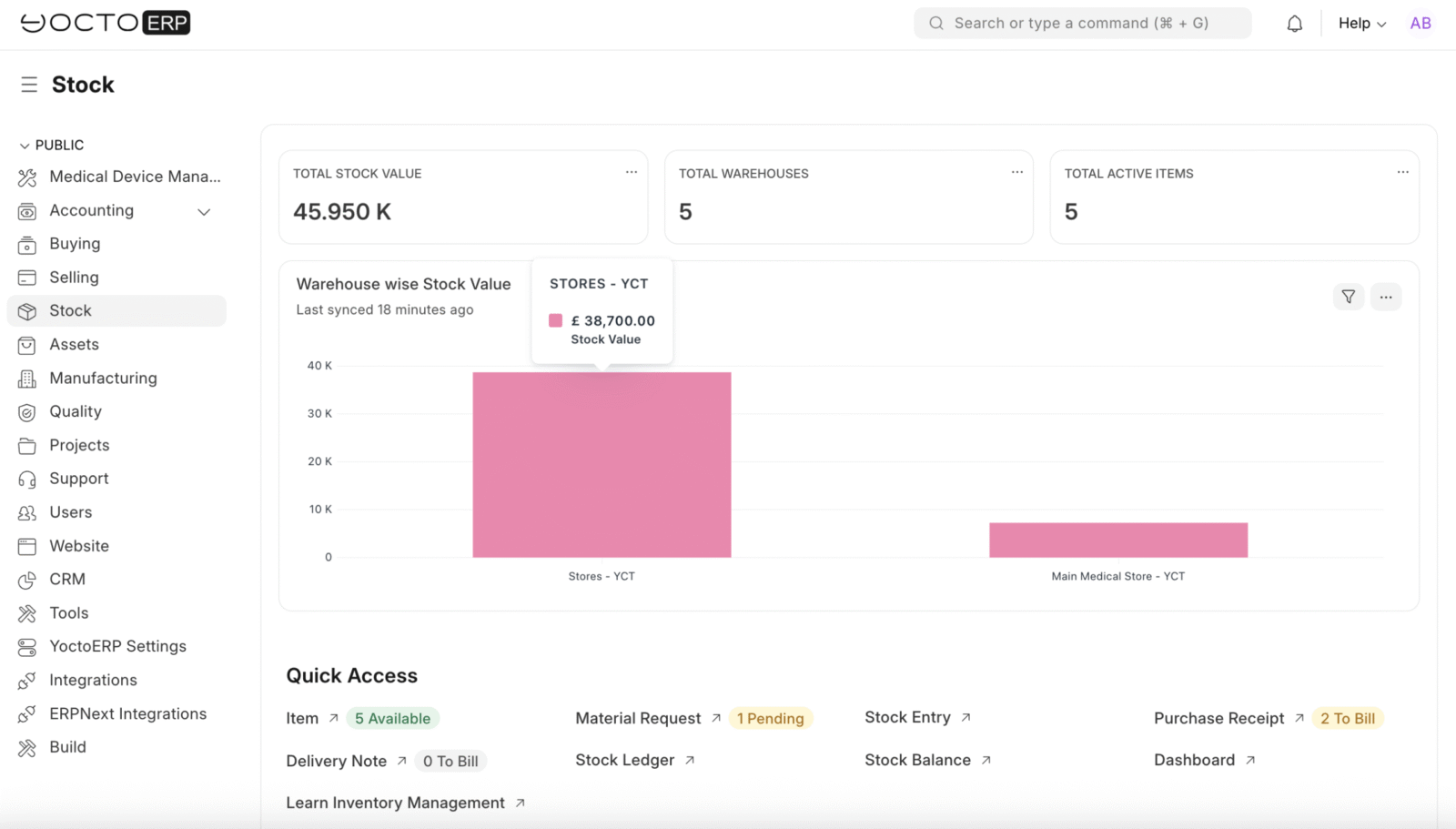
Inventory Management That Works
After inspection, YoctoERP manages stock efficiently:
- Stock Transfers: Move items from quarantine to main storage automatically after approval.
- Real-Time Tracking: See stock levels, batch numbers, and device locations at a glance.
- Reporting: Generate movement, inspection, and compliance reports in minutes, not days.
For medical device companies, this is more than convenience—it’s a regulatory requirement.
End-to-End Compliance Documentation
Regulatory bodies require detailed records for every device batch. YoctoERP keeps all documents in one place:
- Supplier certificates and delivery notes
- Quality inspection results
- Batch tracking and stock movements
Every action is timestamped, linked, and searchable. This ensures you can respond immediately to audits or recalls.
Real-World Impact
Consider a mid-size medical device company managing thousands of units of diagnostic devices across multiple warehouses. Before YoctoERP, stock discrepancies and missing inspection certificates delayed shipments, increased audits, and caused customer dissatisfaction. With YoctoERP:
- Quality inspections are automated and consistent.
- Inventory is accurate and traceable in real time.
- Regulatory compliance is maintained without extra administrative work.
The result: fewer errors, faster operations, and improved compliance.
Why YoctoERP is Different
Most ERPs are generic and require extensive customization for medical device companies. YoctoERP is built for this industry:
- Pre-configured workflows for procurement, goods receipt, and quality inspection
- Templates for medical device compliance checks
- Traceable stock and batch management
- Seamless audit-ready reporting
It’s not theory—it’s a ready-to-use system for teams that need accuracy and efficiency from day one.
Bottom Line
Medical device companies can’t compromise on compliance or efficiency. YoctoERP provides a single platform for procurement, inventory, quality inspections, and regulatory documentation. It reduces errors, saves time, and ensures your devices reach patients safely and legally.
If you want to eliminate manual paperwork, streamline operations, and maintain regulatory compliance, YoctoERP is the medical device ERP your company needs.