Goods Receipt and Quality Inspection Process
Overview
This tutorial covers the critical receiving and quality inspection process for medical devices. You’ll learn to receive goods against purchase orders, perform mandatory quality inspections, handle compliance documentation, and update inventory. This process ensures regulatory compliance and maintains product quality standards.
Prepare for Goods Receipt
Current Status Check
You should have these Purchase Orders ready:
- PUR-ORD-2025-00001: MedTech Solutions Ltd – £7,250.00
- PUR-ORD-2025-00002: European Medical Devices AG – £8,250.00
Setup Quality Inspection Templates
Before receiving goods, set up inspection templates for medical devices:
- Search: Quality Inspection Template
- Click New
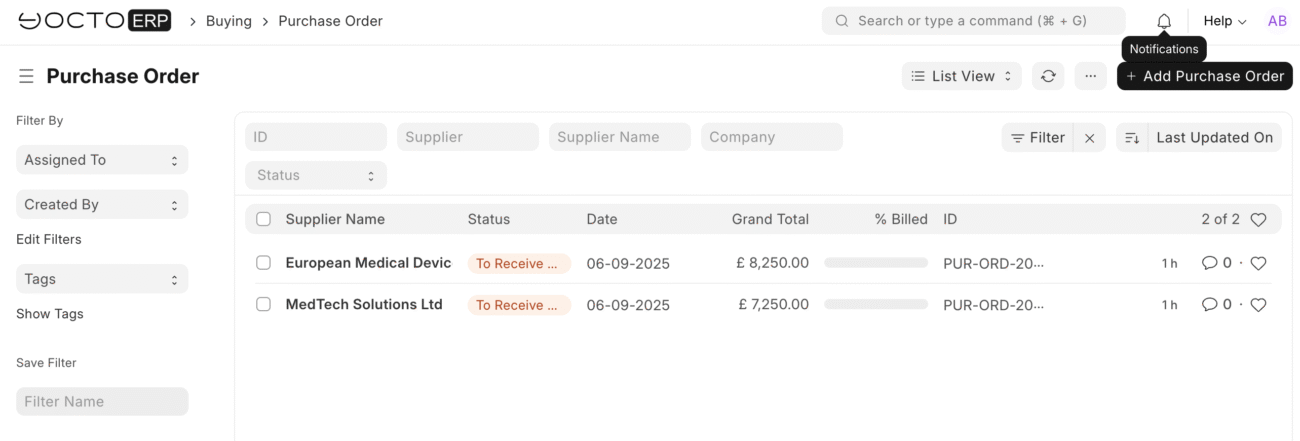
Template 1: Pulse Oximeter Inspection
- Template Title: Medical Device – Pulse Oximeter
- Item Code: POX-001
- Quality Inspection Template Name: PULSE-OX-INSPECT
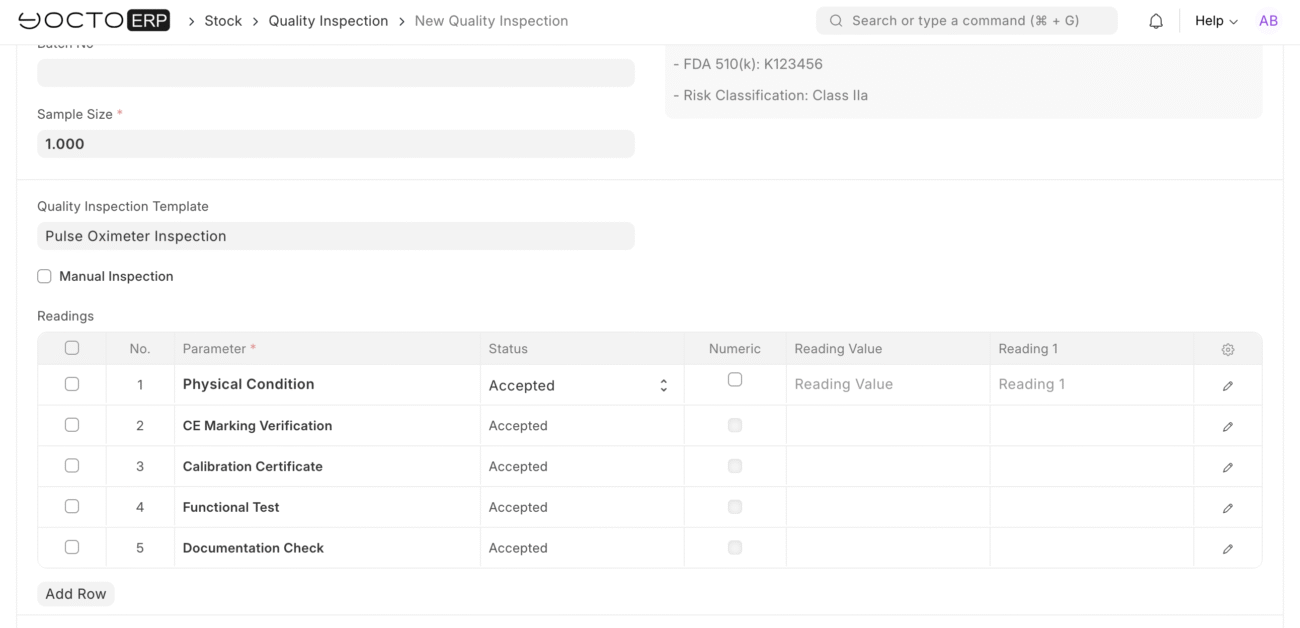
Inspection Parameters: Add these quality parameters:
Parameter 1:
- Specification: Physical Condition
- Acceptance Criteria: No damage, complete accessories
Parameter 2:
- Specification: CE Marking Verification
- Acceptance Criteria: Valid CE mark visible and legible
Parameter 3:
- Specification: Calibration Certificate
- Acceptance Criteria: Current calibration certificate provided
Parameter 4:
- Specification: Functional Test
- Acceptance Criteria: Device powers on and displays readings
Parameter 5:
- Specification: Documentation Check
- Acceptance Criteria: User manual and warranty card included
Save and Submit
Create Purchase Receipt for MedTech Solutions
Receive First Delivery
- Open: PUR-ORD-2025-00001 (MedTech Solutions)
- Click: Create → Purchase Receipt
- Auto-populated data: Items, quantities, rates from PO
Purchase Receipt Details
Receipt Header:
- Supplier: MedTech Solutions Ltd
- Company: [Your Company]
- Posting Date: Today’s date
- Posting Time: Current time
- Set Warehouse: Quarantine Store – [Company] (not Main Medical Store yet)
Why Quarantine Store? Medical devices must be quality inspected before being available for use.
Items Received
The items should auto-populate from the Purchase Order:
Item Details:
- Item Code: POX-001
- Description: Digital Pulse Oximeter – Model PX100
- Received Qty: 50
- Rate: £145.00
- Amount: £7,250.00
- Warehouse: Quarantine Store – [Company]
Delivery Note Details
Additional Information:
- Supplier Delivery Note: DN-MEDTECH-001
- Bill of Lading: BL-UK-2025-789
- Transporter: DHL Medical Logistics
- Vehicle Number: MED-TRANS-456
Quality Inspection
Make sure inspection required before purchase is checked in stock items, and select the appropriate Quality Inspection Template
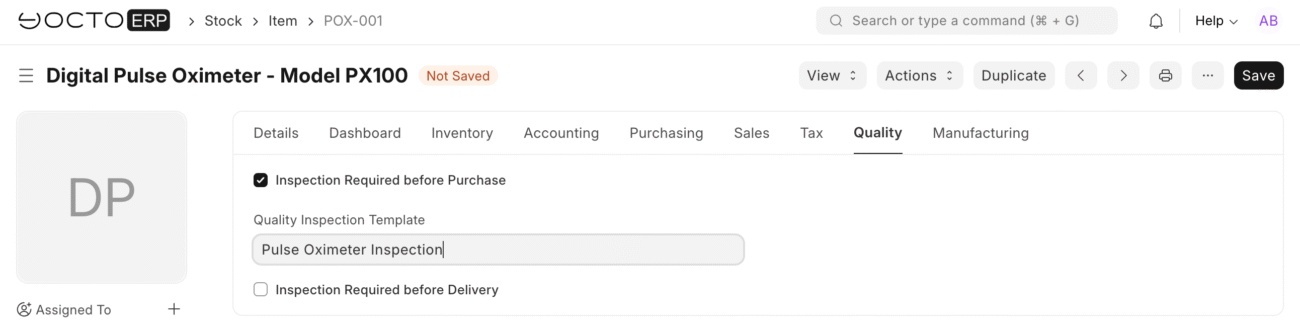
- Navigate to quality inspection list > Create new
- Quality Inspection Template: Select “Medical Device – Pulse Oximeter”
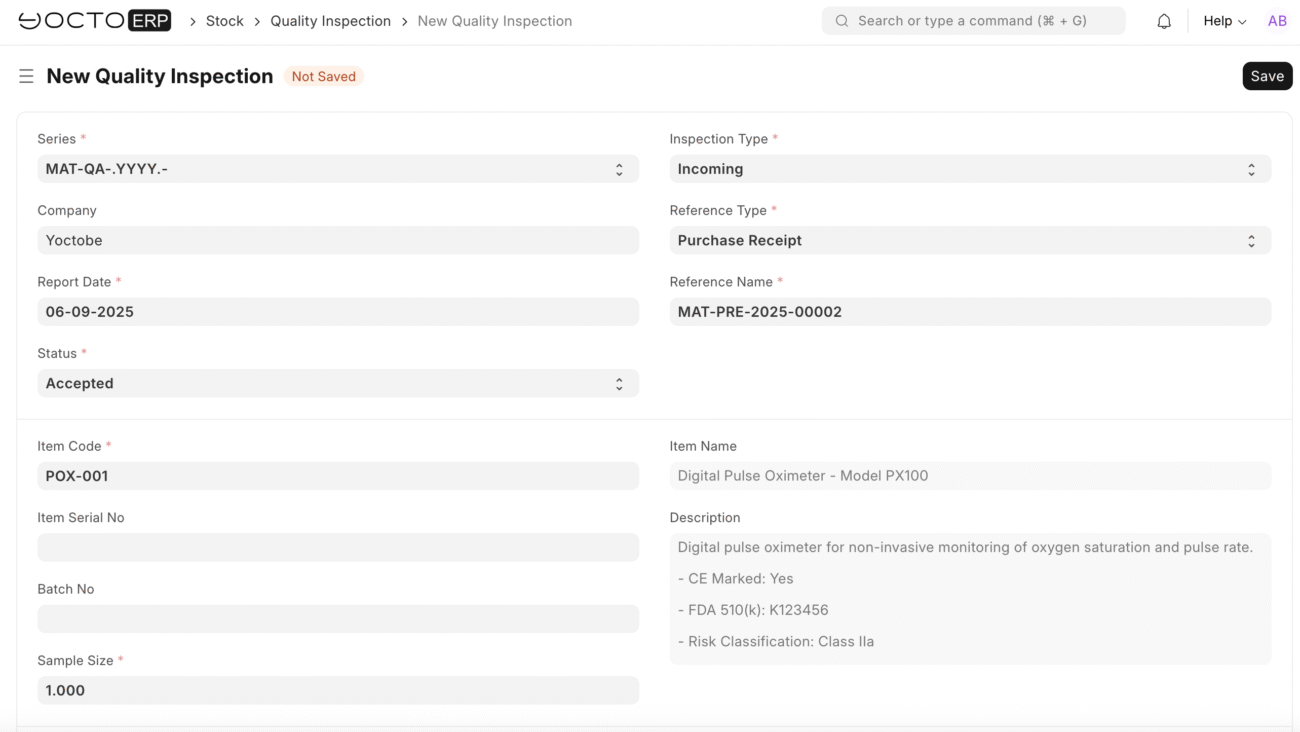
Save and Submit
- Save the Purchase Receipt
- Submit the Purchase Receipt
- Note: Stock goes to Quarantine Store, not available for use yet
Create Purchase Receipt for European Medical Devices
Receive Second Delivery
- Open: PUR-ORD-2025-00002 (European Medical Devices AG)
- Click: Create → Purchase Receipt
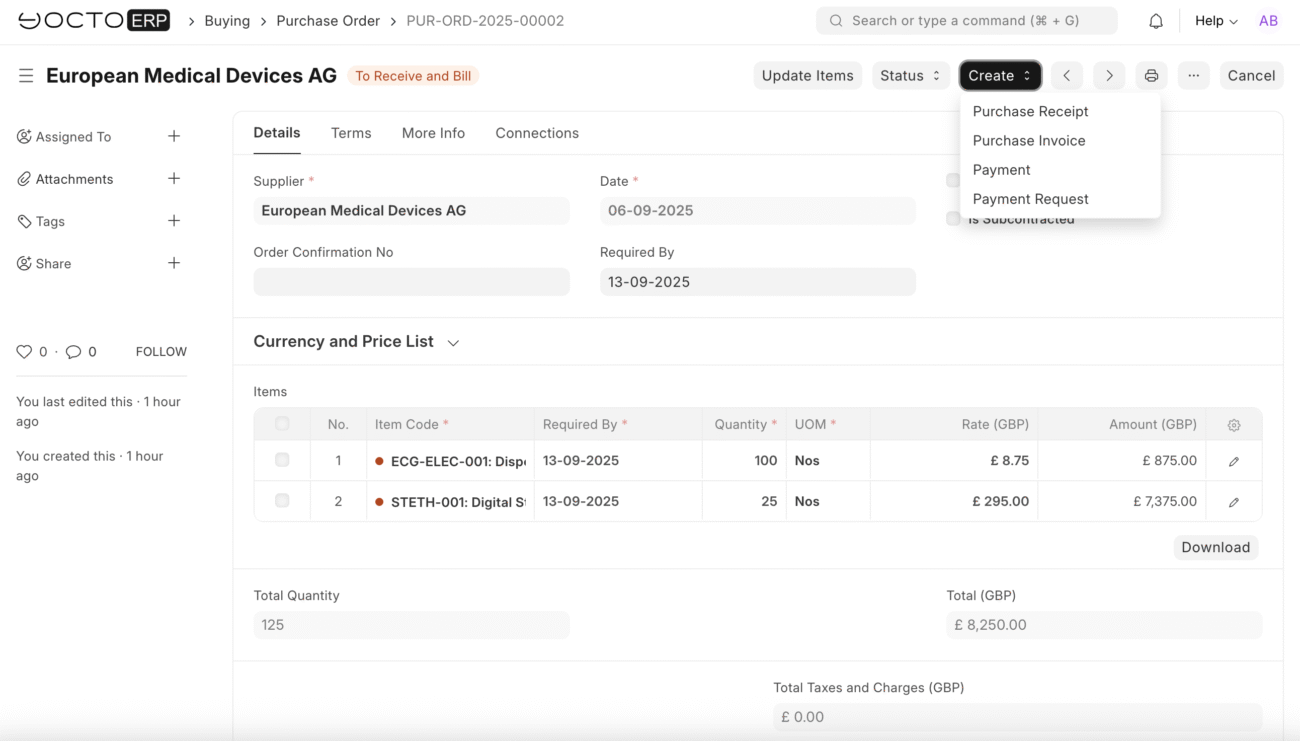
Purchase Receipt Details
Receipt Header:
- Supplier: European Medical Devices AG
- Posting Date: Today’s date
Items Received
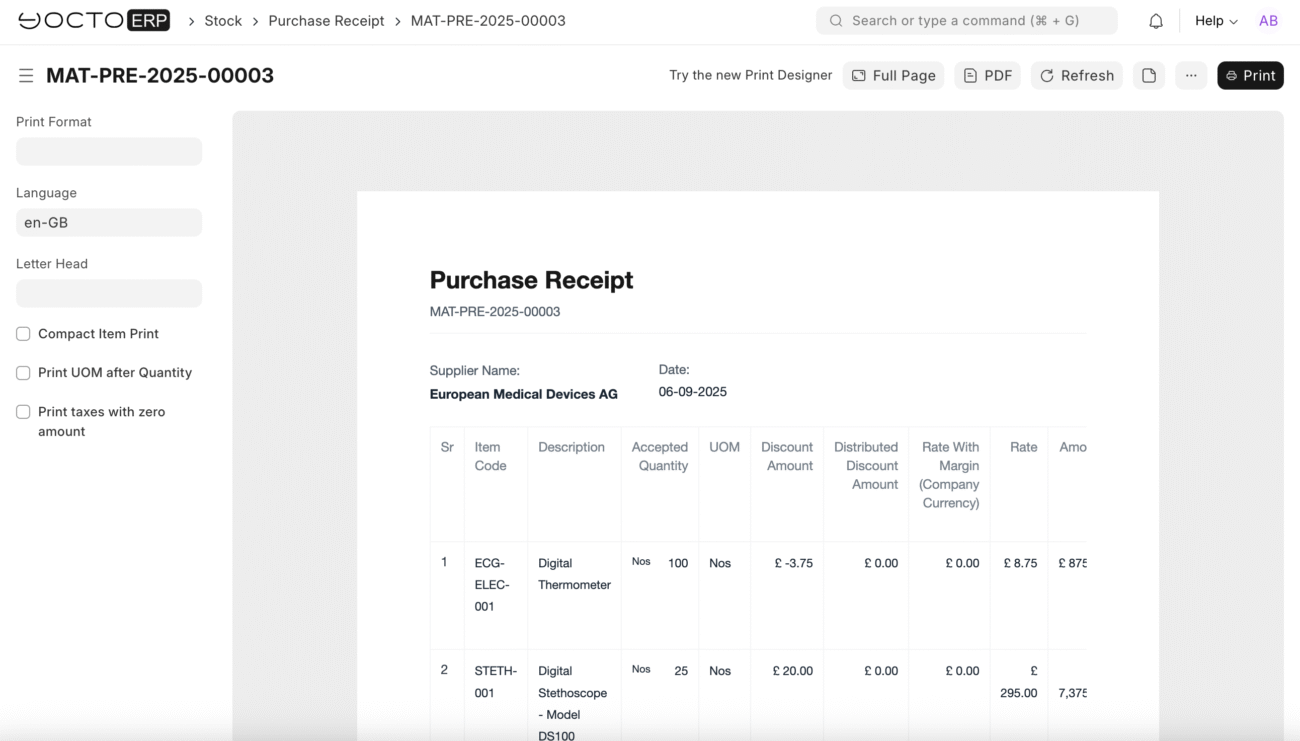
Item Details:
- Item Code: STETH-001
- Description: Digital Stethoscope – Model DS100
- Received Qty: 25
- Rate: £295.00 (note: this differs from tutorial example – using your actual rate)
- Amount: £7,375.00
You can print the purchase receipt
Create Quality Template for Stethoscope
Same steps
Perform Quality Inspections
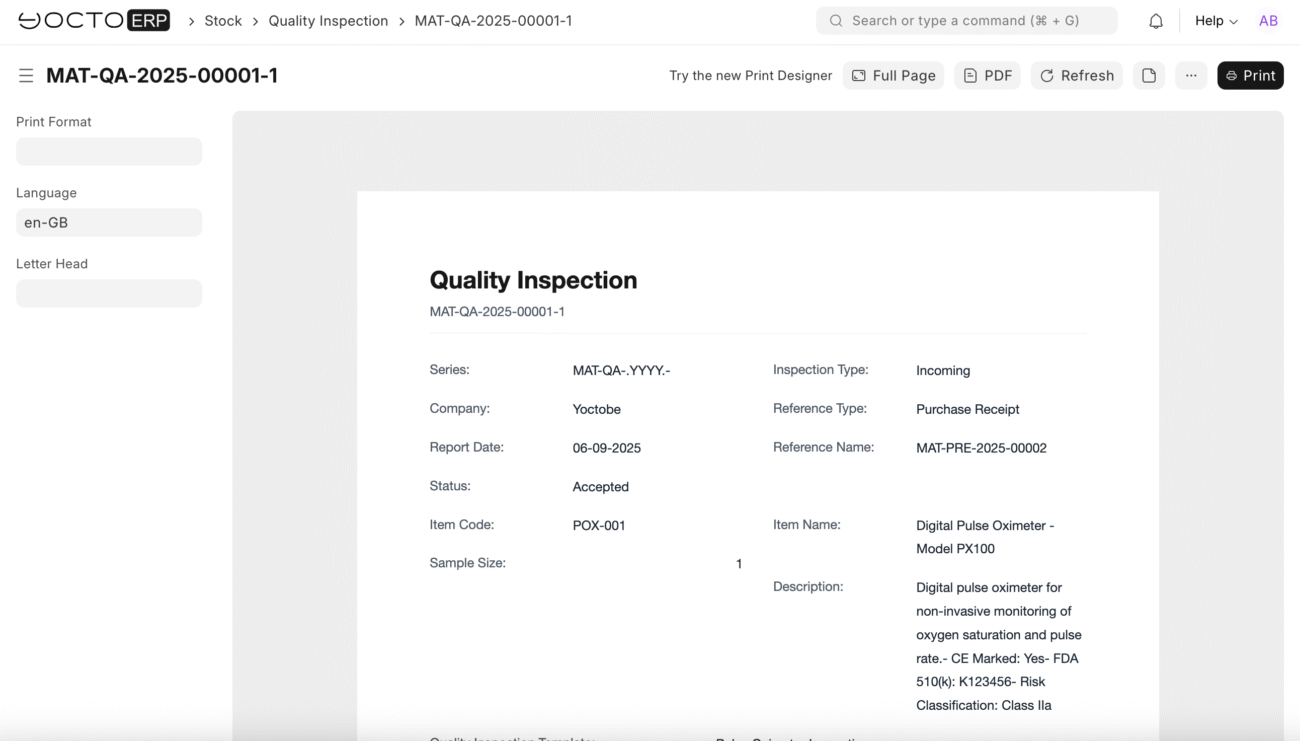
Quality Inspection 1: Pulse Oximeters
- Search: Quality Inspection
- Click New or from Purchase Receipt, click Create → Quality Inspection
Inspection Header:
- Inspection Type: Incoming
- Reference Type: Purchase Receipt
- Reference Name: [Your Purchase Receipt number]
- Item Code: POX-001
- Sample Size: 5 (inspect 5 out of 50 units)
- Inspected By: [Your QC Manager name]
- Inspection Date: Today’s date
Inspection Results
Parameter Testing:
Physical Condition
- Specification: Physical Condition
- Reading 1: Pass
- Reading 2: Pass
- Reading 3: Pass
- Status: Accepted
CE Marking Verification
- Specification: CE Marking Verification
- Reading 1: Pass – CE mark clearly visible
- Reading 2: Pass – Notified body number present
- Reading 3: Pass – Declaration of conformity provided
- Status: Accepted
Calibration Certificate
- Specification: Calibration Certificate
- Reading 1: Pass – Certificate dated within 6 months
- Reading 2: Pass – Calibration traceable to national standards
- Status: Accepted
Functional Test
- Specification: Functional Test
- Reading 1: Pass – SpO2 reading 98% (normal)
- Reading 2: Pass – Pulse rate 72 BPM (normal)
- Reading 3: Pass – All display functions working
- Status: Accepted
Documentation Check
- Specification: Documentation Check
- Reading 1: Pass – User manual included
- Reading 2: Pass – Warranty card completed
- Reading 3: Pass – Quick reference guide included
- Status: Accepted
Overall Inspection Result
- Overall Status: Accepted
- Quality Inspection Summary: All 50 units approved for use. Full compliance with medical device standards.
- Remarks: Batch number: POX-2025-Q3-001. Expiry date: September 2027.
Save and Submit
Quality Inspection 2: Digital Stethoscopes
Create second Quality Inspection:
Inspection Header:
- Item Code: STETH-001
- Sample Size: 3 (inspect 3 out of 25 units)
- Inspected By: [QC Manager name]
Inspection Results:
Physical Condition: Accepted – No physical damage, complete with chest piece covers CE Marking: Accepted – Valid CE marking and documentation Audio Quality: Accepted – Clear heart and lung sound transmission
Battery Check: Accepted – Full charge, 8-hour battery life confirmed Documentation: Accepted – Complete user manual and calibration certificate
Overall Status: Accepted Remarks: Batch STETH-2025-EU-15. All units meet ISO 27614 standards.
Save and Submit
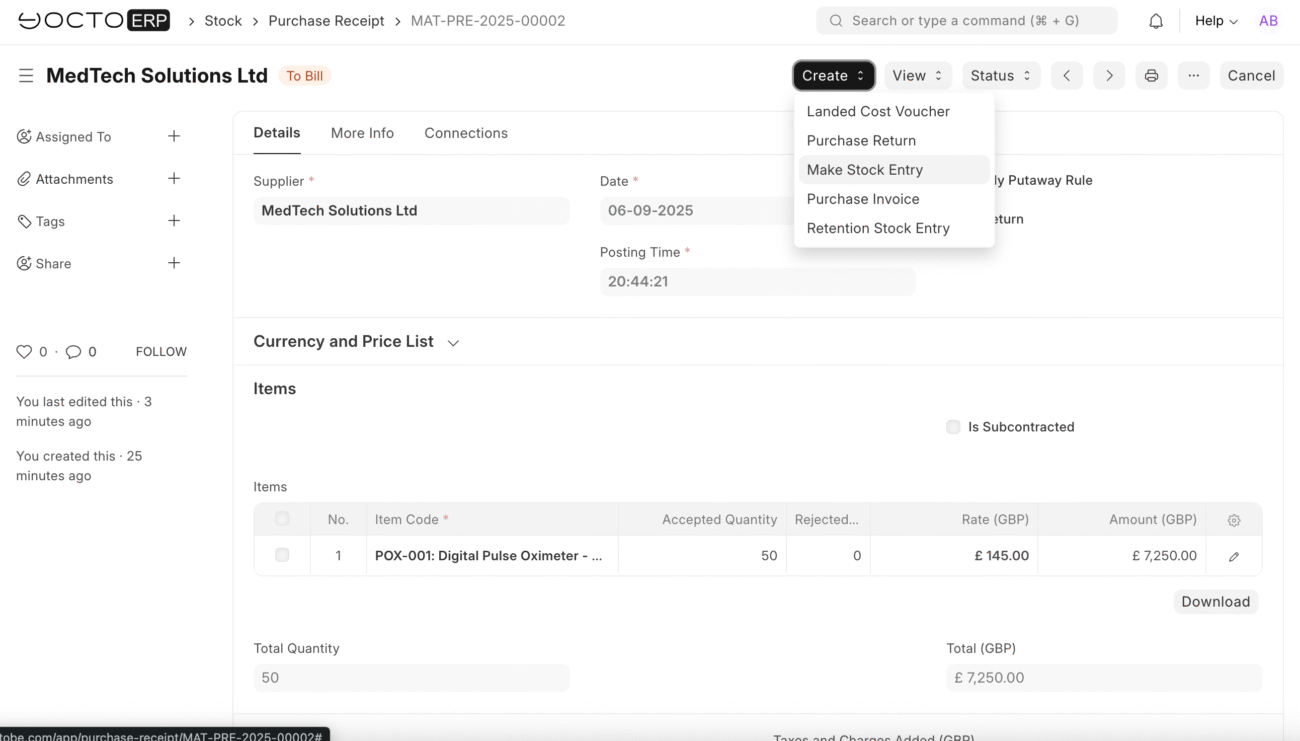
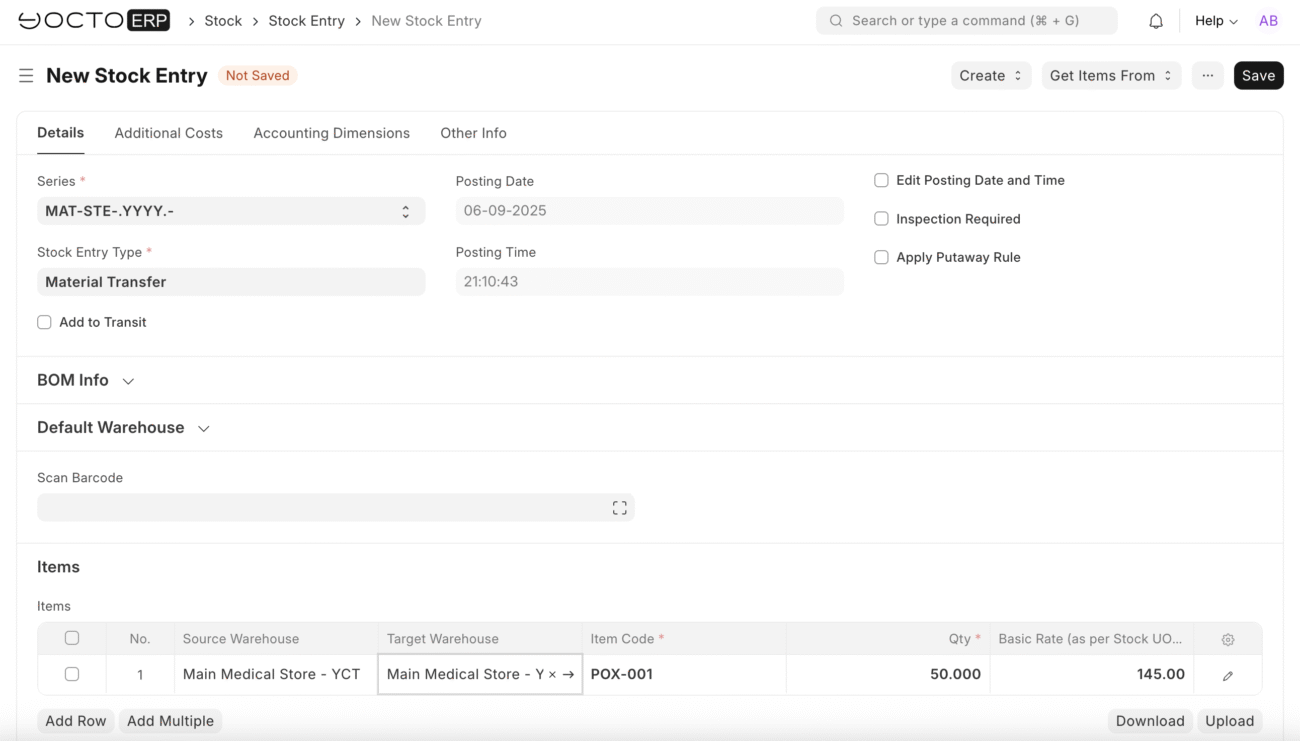
Stock Entry
Transfer Approved Items
After successful quality inspections, transfer accepted items to the target warehouse store:
Handle Partial Delivery (Optional Advanced Scenario)
Simulate Real-World Scenario
Let’s simulate that only 20 stethoscopes arrived instead of 25:
- Amend the European Medical Purchase Receipt
- Change Received Qty from 25 to 20
- Update Amount to £5,900.00 (20 × £295.00)
- Add Note: “Partial delivery – remaining 5 units to follow”
Short Delivery Documentation
Create Delivery Discrepancy Note:
- Search: Note (or create custom document)
- Title: Delivery Discrepancy – PUR-ORD-2025-00002
- Content:
Delivery Discrepancy Report
- Purchase Order: PUR-ORD-2025-00002
- Supplier: European Medical Devices AG
- Ordered Quantity: 25 units
- Received Quantity: 20 units
- Short Delivery: 5 units
- Reason: Temporary stock shortage at supplier
- Expected Delivery: Within 7 days
- Action: Partial acceptance, remaining quantity back-ordered
Update Purchase Order Status
Check PO Status Updates
- View Purchase Order List
- Status should now show:
- PUR-ORD-2025-00001: “To Bill” (fully received)
- PUR-ORD-2025-00002: “To Receive and Bill” (partial delivery)
Handle Back Order (if applicable)
For the short delivery:
- Purchase Order remains open for remaining 5 units
- Create follow-up with supplier
- Set expected delivery date for remaining items
Generate Compliance Reports
Quality Inspection Summary Report
- Search: Quality Inspection Summary
- Set filters: Date range, items, inspector
- Generate report showing all inspections and results
Stock Movement Report
- Search: Stock Ledger
- Filter by: Quarantine Store and Main Medical Store
- View: Movement from quarantine to main store
- Export: For compliance documentation
Goods Received Report
- Search: Purchase Receipt
- Filter by: Date range, suppliers
- Summary: Total value received, pending deliveries
Integration with Regulatory Management
Compliance Documentation
Create compliance folder structure:
Medical Device Batch Records:
- Batch number tracking for each received lot
- Quality inspection certificates
- Calibration certificates from suppliers
- CE marking documentation copies
Supplier Qualification:
- Delivery performance tracking
- Quality performance metrics
- Compliance documentation updates
Traceability Setup
Enable batch tracking:
- Item Master: Enable “Has Batch No” for critical devices
- Batch creation: Automatic during goods receipt
- Batch expiry: Set based on device specifications